Description
This product is a replacement following an FDA rule effective 1/18/2017. The FDA has determined that Powdered Surgeon’s Gloves, Powdered Patient Examination Gloves, and Absorbable Powder for lubricating a surgeon’s glove present an unreasonable and substantial risk of illness or injury. Consequently, FDA is banning these products. We are substituting powdered gloves with comparable non-powdered gloves.
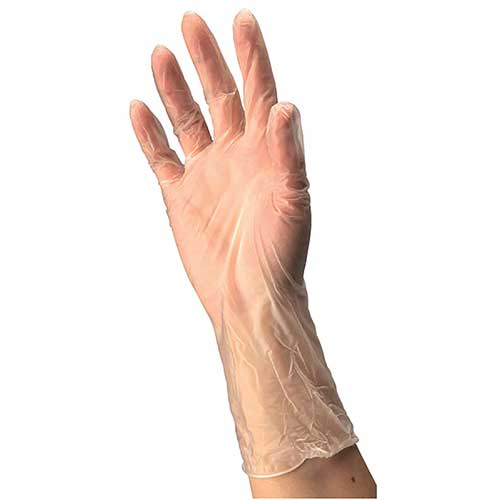
These ambidextrous gloves offer excellent tactile sensitivity and provide maximum dexterity. Gloves meet or exceed ASTM barrier property limits and pass ASTM Bacteriophage Penetration Test F1671.
- Synthetic vinyl provides dexterity
- Beaded cuff facilitates donning
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.